Batteries, just like everything else in life, are just chemistry raised to the power of awesome. The kind of chemistry that happens inside of a battery is called electrochemistry because it involves reactions that produce or consume free electrons. Specifically, they are oxidation or redox reactions, the ones where electrons are exchanged. Now, when the flow of electrons in these kinds of reactions are sent through a conductor, like a piece of metal, it can be used to do all sorts of work. The amount of work that can be done depends on how strong the push or pull on electrons is between the two reactants. This is the reaction’s electrical potential, but to its friends, it’s known simply as voltage. Basically, if the voltage is high, each electron can do a lot more work than if the voltage is low. Many of the wonderful things in our modern lives are based on one simple premise putting a device between the two halves of just such a reaction, the half that donates electrons, and the half that accepts them. Part of what makes redox reactions so powerful, and powerfully excellent, is that they are complicated. Because in each reaction, there are at least two things going on, there’s the part of the reaction where the electrons are being released and another part where they’re being eagerly demanded. So when we deal with electrochemistry, we usually think of reactions in terms of half-reactions.
Let’s take a typical redox reaction that happens in an alkaline battery as an example. In here elemental zinc is going to react with manganese dioxide, also known as manganese four oxide, to produce manganese three oxide and zinc oxide. When you break this down into half-reactions, first you have elemental zinc with an oxidation number of 0 being oxidized to zinc 2 ion. At the same time, manganese 4 is being reduced to manganese 3.
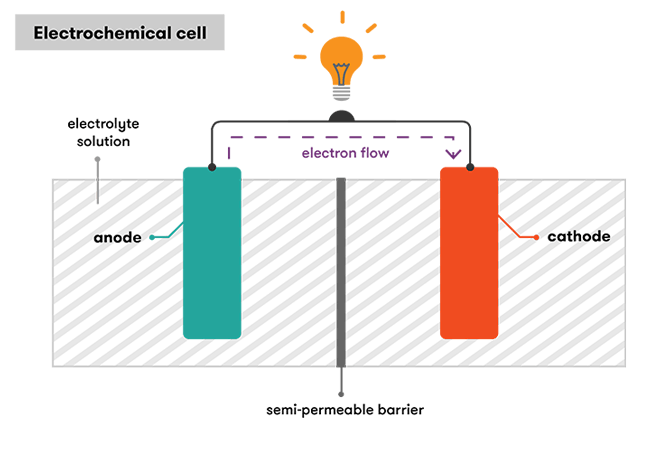
When we balance the half-reactions, we see that two electrons are released during the oxidation of each zinc atom, and one electron is consumed by each manganese 4 atom. The water and hydroxide ions, by the way, come from a solution of potassium hydroxide. Which is a basic, or alkaline compound. This is why we call these things alkaline batteries. Now if each of these half-reactions occurred in contact with the other one, they’d just spontaneously go to equilibrium releasing energy as a bunch of heat which wouldn’t be very helpful. So batteries are designed to harness that energy by isolating the half-reactions from each other. This allows excess electrons to build up in the negative terminal, called the cathode, while an electron vacuum of sorts occurs in the positive terminal, the anode. Electrons can then cross from one-half reaction to the other, only when we connect the cathode and the anode of the battery via conductors. So the current can be used to do work.
In these batteries, the zinc is in the center surrounded by a layer of cellulose that allows ions to pass through. The manganese oxide is in the outer layer that surrounds the zinc core, but the cellulose barrier doesn’t allow the zinc and the manganese to mix. Alkaline batteries are a type of galvanic cell. Which is generally defined as an apparatus that generates electrical energy from a redox reaction.
So, now you know how batteries work, developing and transferring a charge using electrochemistry. The voltage generated by many half-reactions is already known and can be found in most textbooks or online. And as I mentioned earlier, voltage is really just a way of expressing the electrical potential of each half-reaction. The difference between the chemical demand for the electrons in one half, versus the tendency to lose them in the other.
You can use the doubt buddy app to get all your chemistry-related doubts cleared. Not just chemistry you can get help on any subject at any time, instantly.
For more articles from us checkout our blog https://blog.doubtbuddy.com



